Department Of Defense Moderna Vaccine
If you're looking for video and picture information linked to the keyword you've come to visit the right blog. Our website provides you with hints for seeing the maximum quality video and picture content, hunt and find more informative video content and images that match your interests.
includes one of thousands of movie collections from various sources, especially Youtube, therefore we recommend this movie that you view. You can also bring about supporting this site by sharing videos and graphics that you like on this blog on your social networking accounts such as Facebook and Instagram or educate your closest friends share your experiences about the ease of access to downloads and the information that you get on this website. This blog is for them to stop by this site.

Although Slaoui resigned from the Moderna.
Department of defense moderna vaccine. On Friday the Food and Drug Administration announced that it had issued an Emergency Use Authorization EUA for Modernas COVID-19 vaccine the second COVID-19 vaccine to receive an EUA. As the Moderna COVID-19 Vaccine is an mRNA vaccine against COVID-19 encoding for a prefusion stabilized form of the Spike S protein which was co-developed by Moderna and investigators from NIAIDs Vaccine Research Center. Operation Warp Speed has allocated more than 59 million doses of the vaccine for jurisdictions to receive in the coming week. Trump Administration purchases additional 100 million doses of COVID-19 investigational vaccine from Moderna The Trump Administration through the US.
18 the Food and Drug Administration announced that it had issued an Emergency Use Authorization EUA for Modernas COVID-19 vaccine the second COVID-19 vaccine to receive an EUA. The COVID-19 Vaccine Moderna referred to in the US. Dec 29 2020 By Mark Terry The US. Operation Warp Speed has allocated more than 59 million doses of the vaccine for jurisdictions to receive in the coming week.
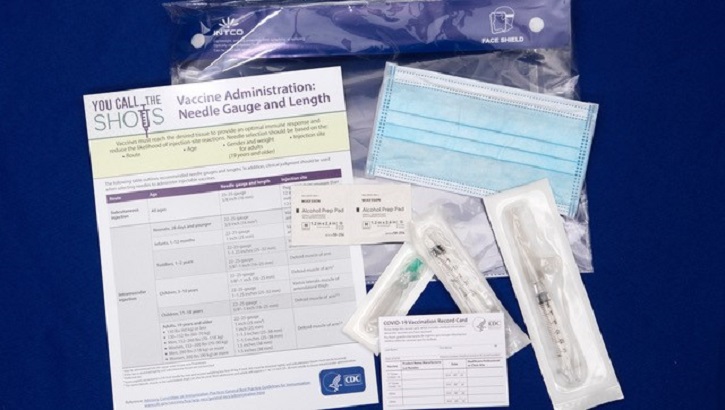
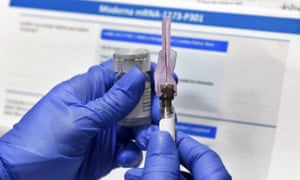
Since December when vaccines from Pfizer-BioNTech and Moderna received Emergency Use Authorization from the Food and Drug Administration the Defense Department has been implementing the phased. Moderna Vaccine Kathryn Cleffi a registered dietician assigned to the 18th Operational Medical Readiness Squadron receives the Moderna COVID-19 vaccine at Kadena Air Base Jan. Brenna Rath receives the first round of the Moderna COVID-19 vaccine at Papago Park Military Reservation in Phoenix Dec. As for the Moderna vaccine earlier this month the company was awarded a 1525 billion contract by the Department of Defense and the Department of Health and Human Services to manufacture and.
Doses of the Moderna COVID-19 vaccine are ready to be administered to airmen of the 163d Attack Wing California Air National Guard at March Air Reserve Base Calif Jan. Department of Health and Human Services HHS and Department of Defense DoD will purchase an additional 100 million doses of COVID-19 vaccine candidate called mRNA-1273 from Moderna. And this week OWS officials expect about 79 million Moderna and Pfizer vaccines to be distributed. Department of Defense contract no.
Vaccine Availability The Department of Defense has announced its deliberate and phased plan to distribute and administer the COVID-19 vaccine to DOD personnel. United States Department of Defense DOD awarded Moderna a contract worth 1966598000 for an additional 100 million doses of its COVID-19 vaccine. To learn more visit. So you have a little bit more flexibility Gilday said.
Drug-maker Moderna has 59-million doses going to 3400 locations and 21-million Pfizer. Is allowed to be refrigerated for up to 30 days. Moderna Vaccine Arizona Army National Guard Spc. Air Force photo by Senior Airman Aaron Irvin.
Coordinate with the Department of Defense for vaccine supply production and deployment around the United States. The COVID-19 Vaccine Moderna referred to in the US. The Moderna COVID-19 Vaccine is given in two doses and is 941 effective according to the Centers for Disease Control and Prevention. Government has agreed to purchase supply of the Moderna COVID-19 Vaccine under US.
Headquartered in Cambridge Mass Moderna currently has strategic alliances for development programs with AstraZeneca PLC and Merck Co Inc as well as the Defense Advanced Research Projects Agency DARPA an agency of the US. A medical technician from the 19th Medical Group administers the Moderna COVID-19 vaccine at Little Rock Air Force Base Arkansas Jan 14 2021. Department of Defense contract no. Government has agreed to purchase supply of the Moderna COVID-19 Vaccine under US.
Moderna is developing therapeutics and vaccines for infectious diseases immuno-oncology rare diseases cardiovascular diseases and autoimmune and inflammatory diseases independently and with strategic collaborators. Moderna has been named a top biopharmaceutical employer by Science for the past six years. The manufacturing will be handled in Cambridge Massachusetts and is expected to be fulfilled by June 30 2021. Moderna has received authorization for its COVID-19 vaccine from regulatory authorities in the United States Canada Israel the European Union the United Kingdom and Switzerland.
As the Moderna COVID-19 Vaccine is an mRNA vaccine against COVID-19 encoding for a prefusion stabilized form of the Spike S protein which was co-developed by Moderna and investigators from NIAIDs Vaccine Research Center. The Moderna COVID-19 vaccine has not been approved or licensed by the FDA or any other health authority but it has been authorized for emergency use by the FDA under an EUA. Vaccine developer Moderna and divested his shares in Moderna stock at a potential personal gain of 10 million raising questions of his neutrality in judging vaccine candidates.